
Electrode calibration is necessary in order to establish the slope and zero point of the electrode. Since both of these can change over time, frequent calibration is necessary. The frequency of calibration depends on the application, with some applications requiring daily calibration while others may require only weekly or monthly calibration. More frequent calibration is recommended when measuring in heavily contaminated, low-ion, strongly acidic, and high temperature solutions. The following is a general procedure for preparing most pH electrodes.
Perform Routine Maintenance
- On a weekly basis, inspect the pH electrode for scratches, cracks, salt crystal build-up, or membrane/junction deposits.
- Keeping an electrode clean can help eliminate calibration issues. Clean any salt deposits from the electrode exterior by rinsing it with distilled water before use. Always check the meter and electrode manuals for calibration and routine maintenance information.
- Place the electrode for 10 minutes in 0.1 M HCl or 0.1 M NaOH. If the buildup is not removed, the solution should be cautiously heated up to 45 o C - 55 o C for 10 minutes before the acid or alkaline concentration is increased.
Open the Refill Slider/Ring
- For pH electrodes featuring a refillable reference, the first step to calibrating and/or taking a measurement is to open the refill opening. Depending on the model, the refill opening is either a slider (left image) or a ring (right image). The refilling opening must always be open during calibration and measurement.
Check the Electrolyte Level
- For refillable electrodes, ensure the fill level of the electrolyte is at least 2 cm above the level of the measurement solution. Replace the electrolyte if it has become contaminated.
Check the Selected Buffer Set
- The pH values of buffer solutions are temperature dependent and the response can vary from manufacturer to manufacturer. Also, the pH values of buffers in a buffer set can vary from one set to another. Modern pH meters automatically adjust for the respective temperature profile once the buffer set used has been correctly set.
Use Fresh, Unused, Unexpired Buffers
- Once buffers are used for calibration, they are assumed contaminated and should not be used
 again. Reusing buffers can lead to slow pH electrode response or the inability to calibrate. The cause of calibration failure is difficult to determine if the pH buffers have already been used. Used buffer solutions can be kept for rinsing the calibration container and the electrode between calibration points.
again. Reusing buffers can lead to slow pH electrode response or the inability to calibrate. The cause of calibration failure is difficult to determine if the pH buffers have already been used. Used buffer solutions can be kept for rinsing the calibration container and the electrode between calibration points.
- Expired buffer solutions should not be used and buffer bottles should not be left open. Carbon dioxide in the air can change the pH of basic buffer solutions, so basic buffer bottles should only briefly be opened. Use opened containers of buffer as soon as possible.
- Expiration dates are printed on the label of the buffer bottle, and according to the LOT code
visit ohaus.com/Lot-Certificates
Click on the images to learn more about each Buffer
Buffer pH10.01 250 ml

|
Buffer pH12.45 250 ml

|
Buffer pH1.68 250 ml

|
Buffer pH4.01 250 ml

|
Buffer pH6.86 250 ml
 |
Buffer pH7.00 250 ml

|
Buffer pH9.18 250 ml

|
All Water Analysis Solutions
 |

|
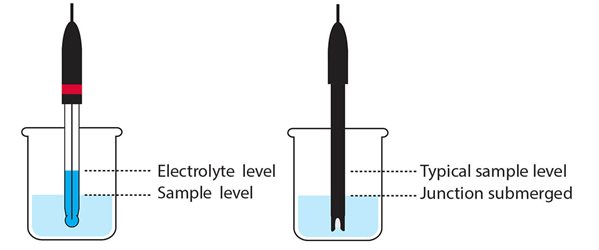
The Reference Junction Should be Immersed
- The reference junction must be completely submerged in solution. The temperature sensor must also be in solution in order to accurately compensate pH for temperature.
- The sample solution level must be above the pH electrode reference junction when the electrode is immersed in the sample.
Perform at Least a 2-Point Calibration
- It is best to perform at least a 2-point calibration and pH 7 buffer must be one of those points.
- The pH buffers used should differ by at least two pH units and should bracket the expected in situ pH conditions. Calibration points need to bracket your sample range. Unless the sample is expected to be above pH 7, basic buffers should not be used, as their pH value quickly changes by absorbing CO2
- When measurements are performed over a large range of pH values, it is recommended that one takes at least 3 calibration points. A 1-point calibration will only determine the zero point, not the electrode slope. The range of use of 1-point calibrations is limited and should only be completed with pH 7 buffer. The pH value obtained can be used to compare to previous results, but is not an absolute value.
- Between buffers, rinse the electrode with distilled water and then with the next buffer. To reduce the chance of error due to polarization, avoid rubbing or wiping the electrode bulb. Use a lint-free tissue and gently blot the bulb.
- The first calibration point should be pH 7. Although it is not always required, it is best to begin calibration with pH 7 buffer.
Learn more about OHAUS Electrochemistry products at:
Water Analysis Meters & Electrodes